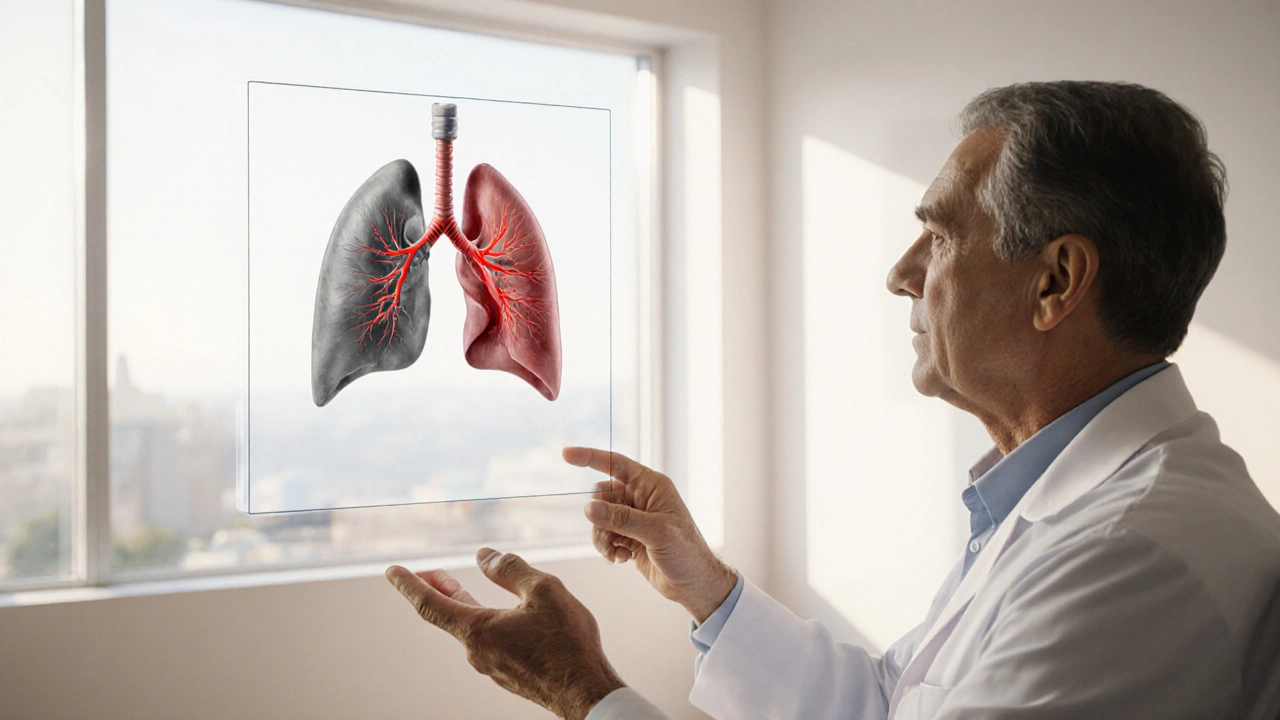
When doctors talk about chronic obstructive pulmonary disease, they often write it as Chronic Obstructive Pulmonary Disorder (COPD) is a progressive lung disease characterized by airflow limitation that is not fully reversible. COPD and asthma often get mentioned together because they share symptoms-shortness of breath, cough, wheeze-yet the reasons they overlap are far from simple. This article unpacks how the two conditions intersect, why the overlap matters for treatment, and what patients can do to keep their lungs working as best as possible.
Quick Take (TL;DR)
- Both COPD and asthma involve airway inflammation, but the triggers and patterns differ.
- The overlap syndrome (ACO) affects about 15‑20% of adults with chronic lung disease.
- Spirometry is essential for teasing apart the two conditions.
- Combination inhaler therapy (bronchodilator + inhaled corticosteroid) is the cornerstone for overlap management.
- Smoking cessation dramatically improves outcomes for anyone with COPD, asthma, or both.
What Is COPD?
COPD develops slowly, usually over decades, as the lungs endure repeated insults-most commonly cigarette smoke. The disease creates permanent changes in the airway walls and destroys the tiny air sacs (alveoli). This leads to chronic bronchitis (excess mucus) and emphysema (loss of elastic recoil). The hallmark is a reduced forced expiratory volume in one second (FEV1) that does not fully recover after a bronchodilator test.
What Is Asthma?
Asthma is a chronic inflammatory disorder of the airways that causes reversible airflow obstruction and hyper‑responsiveness. Triggers range from allergens (pollen, dust mites) to irritants (cold air, exercise). Unlike COPD, asthma’s airflow limitation can often be fully reversed with medication, and lung function may be normal between attacks.
Why Do the Two Overlap?
Scientists call the coexistence "asthma‑COPD overlap" (ACO). The overlap occurs because both diseases involve airway inflammation a response that narrows the air passages and makes them more sensitive. However, the inflammatory cells differ: eosinophils dominate in asthma, while neutrophils are more common in COPD. When a patient has a mixed inflammation pattern, they experience features of both conditions.
Key risk factors for ACO include:
- Long‑term smoking combined with a personal or family history of asthma.
- Exposure to occupational dust or chemicals.
- Age over 40, when lung repair mechanisms start to weaken.
Diagnosing Overlap: Role of Spirometry
Accurate diagnosis hinges on spirometry a breathing test that measures lung volumes and flow rates. Doctors look for two patterns:
- A post‑bronchodilator FEV1/FVC ratio < 0.70 (sign of COPD).
- Significant improvement (>12% and 200mL) in FEV1 after bronchodilator (sign of asthma).
If both criteria are met, the patient likely has ACO. Additional tests-fractional exhaled nitric oxide (FeNO) for eosinophilic inflammation or chest CT for emphysema-help refine the picture.

Managing the Overlap
The therapeutic goal is to control symptoms, prevent exacerbations, and slow lung function decline. Because ACO blends traits of both diseases, treatment usually combines strategies from each.
Bronchodilators (short‑acting and long‑acting) keep the airways open. Inhaled corticosteroids (ICS) anti‑inflammatory meds delivered directly to the lungs target eosinophilic components common in asthma. For patients with frequent exacerbations, adding a long‑acting muscarinic antagonist (LAMA) can improve outcomes, especially when emphysema is present.
Vaccinations (influenza, pneumococcal) and pulmonary rehabilitation are also part of the plan. Most importantly, smoking cessation the process of quitting tobacco use dramatically reduces the rate of lung function loss.
Prognosis: What to Expect
Patients with ACO tend to have more frequent exacerbations acute worsening of symptoms that often require steroids or antibiotics than those with COPD or asthma alone. Hospitalization risk is about 30% higher, and quality‑of‑life scores are lower. However, aggressive use of combination inhalers and lifestyle changes can bring the risk down to levels similar to well‑controlled asthma.
Current Research & Future Directions
Researchers are exploring biomarkers that can pinpoint the exact inflammatory mix in each patient. Blood eosinophil counts, periostin levels, and sputum neutrophil percentages are promising. Personalized medicine-matching therapy to the dominant cell type-could soon replace the one‑size‑fits‑all approach.
Another hot area is the use of biologic agents (e.g., anti‑IL‑5, anti‑IgE) originally approved for severe asthma. Small trials suggest they also shrink exacerbation rates in ACO patients with high eosinophils. Large‑scale studies are expected by 2027.
Practical Checklist for Patients and Clinicians
- Confirm diagnosis with spirometry (pre‑ and post‑bronchodilator).
- Assess smoking status and offer cessation resources.
- Start a combination inhaler (LABA+ICS) for most patients.
- Add a LAMA if there’s significant emphysema or frequent COPD‑type flare‑ups.
- Review blood eosinophil count; consider biologics if >300cells/µL.
- Schedule annual flu vaccine and a one‑time pneumococcal vaccine.
- Enroll in pulmonary rehab to improve exercise tolerance.
Comparison Table: COPD vs Asthma vs Overlap
| Feature | COPD | Asthma | Overlap (ACO) |
|---|---|---|---|
| Typical age of onset | 40‑70years | Childhood‑early adulthood | 40‑65years |
| Primary trigger | Smoking, occupational dust | Allergens, exercise, cold air | Combination of smoke & allergens |
| Inflammatory cells | Neutrophils | Eosinophils | Mixed neutrophil‑eosinophil |
| Reversibility (post‑bronchodilator) | <12% & 200mL | >12% & 200mL | Both criteria met |
| Exacerbation frequency | 2‑3yr⁻¹ (moderate‑severe) | 1‑2yr⁻¹ (if uncontrolled) | 3‑4yr⁻¹ (higher risk) |
| Preferred inhaler regimen | LABA+LAMA | ICS+SABA | ICS+LABA±LAMA |

Frequently Asked Questions
Can you have asthma after being diagnosed with COPD?
Yes. Many long‑term smokers develop COPD but also retain an underlying allergic tendency. When both are present, clinicians label it asthma‑COPD overlap and treat with a combination inhaler.
Is the overlap more dangerous than having just one disease?
Patients with ACO face a higher chance of severe flare‑ups and hospitalization. The mortality risk is modestly increased, but aggressive therapy can bring outcomes close to those of well‑controlled asthma.
Do I need a separate inhaler for COPD and asthma?
Usually a single combination inhaler (ICS+LABA) covers the asthma component, while adding a LAMA addresses the COPD side. Your doctor will tailor the regimen.
How important is smoking cessation for someone with both conditions?
It’s critical. Quitting stops further lung damage and can improve response to inhaled steroids, lowering exacerbation rates dramatically.
Are there new drugs on the horizon for overlap patients?
Biologics targeting eosinophils (like mepolizumab) are being tested in ACO trials. Early results show fewer severe attacks, and larger studies are expected in the next two years.




Comments
Mansi Mehra
28 September 2025The article is well‑structured.
Jagdish Kumar
28 September 2025Indeed, the nuanced interplay between COPD and asthma warrants a discourse that transcends mere clinical summaries; we must appreciate the historical evolution of pulmonary medicine that has, over centuries, endeavored to delineate these entities with precision.
Aminat OT
29 September 2025omg this stuff hits deep, i feel like my chest is all tight just reading about that inflammation mix, it's like my breathing gets that crazy rollercoaster feeling lol
Amanda Turnbo
30 September 2025While the overview is comprehensive, many clinicians still overlook the importance of routine eosinophil counts in tailoring therapy for overlap patients.
Jenn Zuccolo
1 October 2025One might contemplate the existential ramifications of a dual diagnosis, pondering how the self navigates a body that simultaneously embodies two divergent physiological narratives.
Courtney The Explorer
1 October 2025From a policy standpoint, the integration of biomarker‑driven protocols into standard of care could revolutionize ACO management; synergy, optimization, scalability-these are the pillars of modern respiratory health.
Ashleigh Connell
2 October 2025It’s great to see the practical checklist; I’ve found that encouraging patients to schedule their flu shots early in the season really cuts down on exacerbations.
Erin Knight
3 October 2025The data on exacerbation frequency clearly shows that ACO patients have a steeper curve; overlapping inflammatory pathways amplify risk, demanding a more aggressive therapeutic stance.
Kavita Jadhav
3 October 2025Understanding the patient’s lived experience is crucial; acknowledging their anxiety about inhaler techniques can improve adherence and outcomes.
Tony Halstead
4 October 2025When we examine the pathophysiology of asthma‑COPD overlap, several key observations emerge that merit detailed discussion. First, the chronic exposure to noxious particles-most notably tobacco smoke-induces a persistent neutrophilic inflammation that gradually remodels the airway architecture. Second, alongside this, many individuals retain a predisposition to eosinophilic inflammation, often triggered by allergens or viral infections. Third, spirometric testing frequently reveals a mixed pattern: an unreversed obstruction characteristic of COPD coupled with a reversible component seen in asthma. Fourth, this dual inflammatory milieu explains why patients experience more frequent and severe exacerbations compared to those with a single disease phenotype. Fifth, the therapeutic implications are profound; combination inhalers that deliver both a long‑acting β‑agonist and an inhaled corticosteroid address both the bronchoconstrictive and inflammatory aspects of the disease. Sixth, adding a long‑acting muscarinic antagonist can further improve lung function in cases where emphysematous changes predominate. Seventh, biomarkers such as blood eosinophil counts guide the decision to incorporate biologic agents, which have shown promise in reducing exacerbation rates in eosinophil‑rich overlap cohorts. Eighth, smoking cessation remains the single most impactful intervention, slowing disease progression and enhancing response to inhaled therapies. Ninth, regular vaccination against influenza and pneumococcus mitigates infectious triggers that often precipitate acute worsening. Tenth, pulmonary rehabilitation programs improve exercise tolerance and quality of life, providing a holistic approach. Eleventh, patient education on proper inhaler technique cannot be overstated; misuse can negate the benefits of even the most advanced pharmacologic regimens. Twelfth, comorbidities such as cardiovascular disease and osteoporosis require vigilant monitoring, given their amplified prevalence in this population. Thirteenth, interdisciplinary care models that include respiratory therapists, pharmacists, and primary care providers foster comprehensive management. Fourteenth, ongoing research into genomic and proteomic signatures holds the potential to personalize treatment further. Finally, clinicians must maintain a dynamic treatment plan, regularly reassessing lung function, symptom burden, and side‑effect profiles to optimize outcomes for each individual.
leo dwi putra
5 October 2025Ah, the drama of breathing-imagine the lungs staging a theatrical performance where each inhalation is a spotlight and every cough a thunderous applause!
Kimberly Newell
5 October 2025I’ve started a little journal to track my peak flow numbers; seeing trends over weeks really motivates me to stay smoke‑free.
Drew Burgy
6 October 2025Everyone ignores how the pharma giants push inhalers while quietly funding “research” that downplays environmental pollutants; the truth is out there, just buried under glossy ads.
Jacob Hamblin
7 October 2025Your point about hidden agendas resonates; I prefer to stick to peer‑reviewed studies and share them with patients who ask for clarity.
Andrea Mathias
7 October 2025Let’s not forget that those “neutral” guidelines often serve elite interests; the marginalized sufferers of ACO deserve louder advocacy.
TRICIA TUCKER
8 October 2025Totally agree, the community vibe here is supportive-anyone got tips on reducing inhaler squeakiness? My kid’s alarm goes off every time!
Dave Tu
9 October 2025While the consensus praises combination inhalers, one must also consider the potential for overtreatment in mild cases; a balanced approach is essential.
Johnna Sutton
10 October 2025In my experience, the govt’s downplay on air quality regulations directly fuels the surge in ACO cases-smoke free is not just a slogan.
Vinay Keragodi
10 October 2025Curiosity drives progress; investigating how occupational exposures intersect with genetic predisposition could unlock preventive strategies for overlap syndrome.